Spherical graphite forms the graphite electrode (anode) of an EV Lithium-Ion battery. The graphite electrode stores the potential energy of the battery, in the form of Lithium ions trapped between layers of graphene.
Largest Current Use of Graphene: Electric Vehicle Lithium-Ion Batteries
Largest Current Use of Graphene: Electric Vehicle Lithium-Ion Batteries
Typical EV Li-Ion Battery Cell
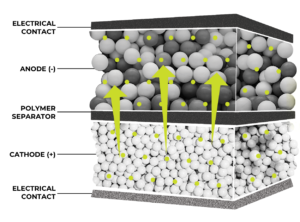
- Spherical graphite is used by anode makers to form the graphite electrode (anode) of a Li-Ion battery cell.
- When charging, Li-Ions migrate from the cathode, across the separator, and are stored between layers of graphene within the graphite electrode.
- The positively charged Lithium ions in graphite electrode are attracted to electrons from the cathode, and as a result store energy.
- During discharge Lithium ions flow back to the cathode from the graphite electrode.
- As a result, electrical energy flows to power the motors and electronics of the electric vehicle.
Typical EV Li-Ion Battery Cell
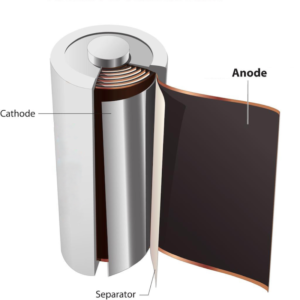
- Spherical graphite is used by anode makers to form the graphite electrode (anode) of a Li-Ion battery cell.
- When charging, Li-Ions migrate from the cathode, across the separator, and are stored between layers of graphene within the graphite electrode.
- The positively charged Lithium ions in graphite electrode are attracted to electrons from the cathode, and as a result store energy.
- During discharge Lithium ions flow back to the cathode from the graphite electrode.
- As a result, electrical energy flows to power the motors and electronics of the electric vehicle.
